Rituximab Moa
These symptoms may start gradually and get worse quickly.
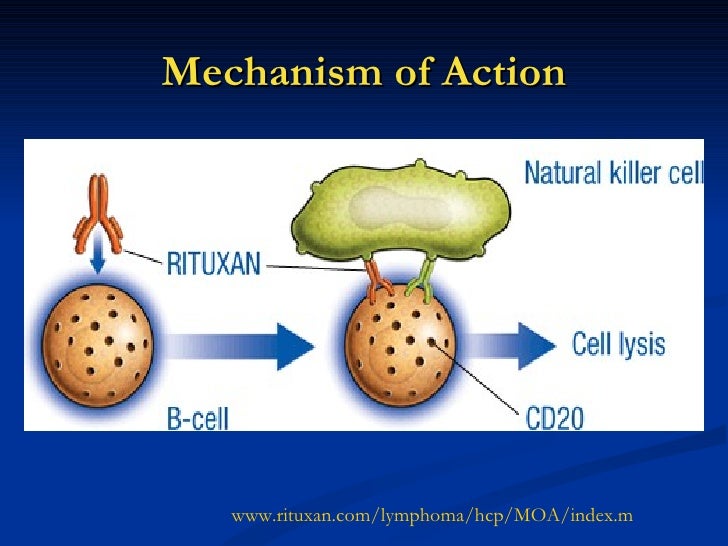
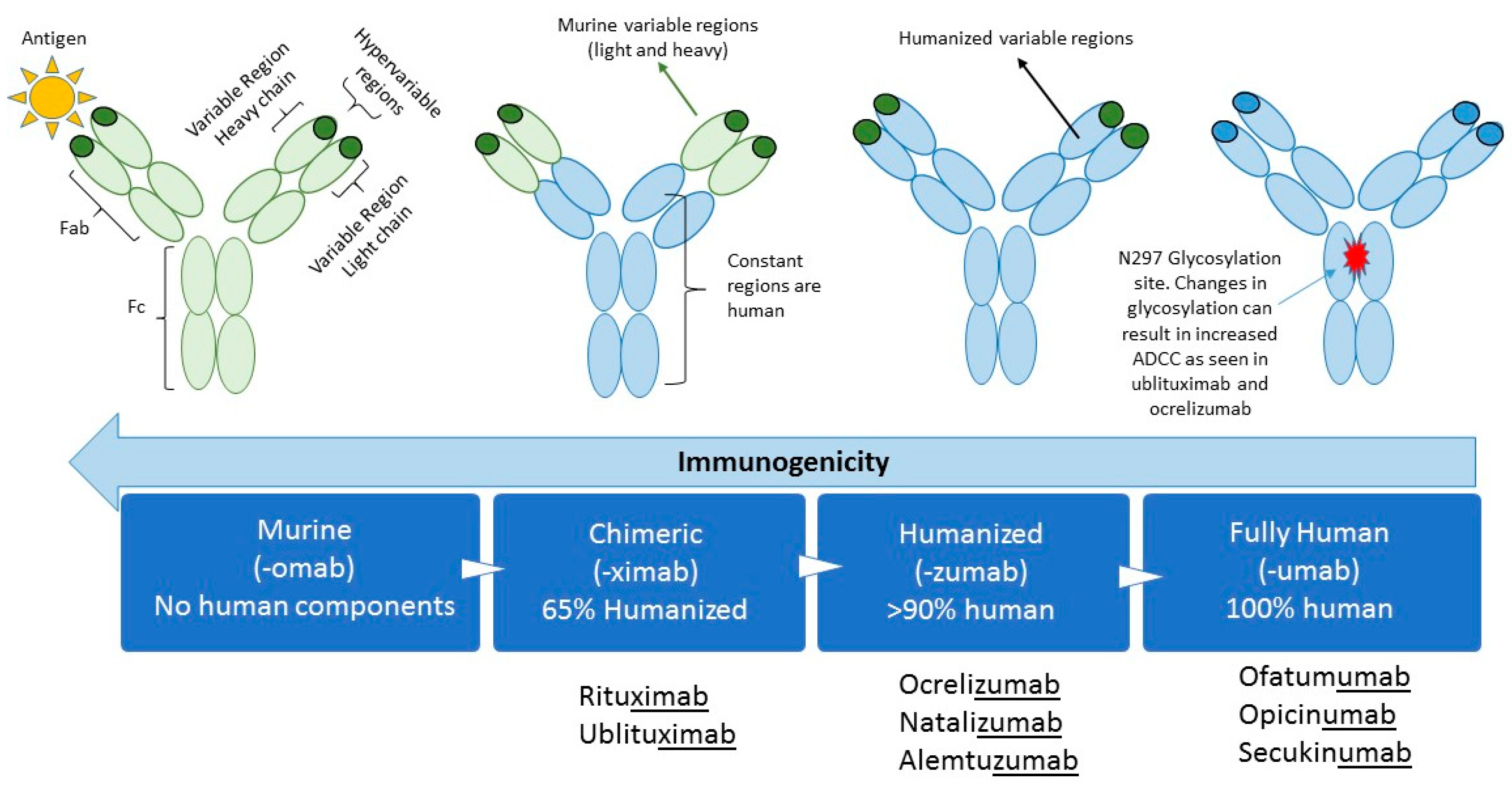
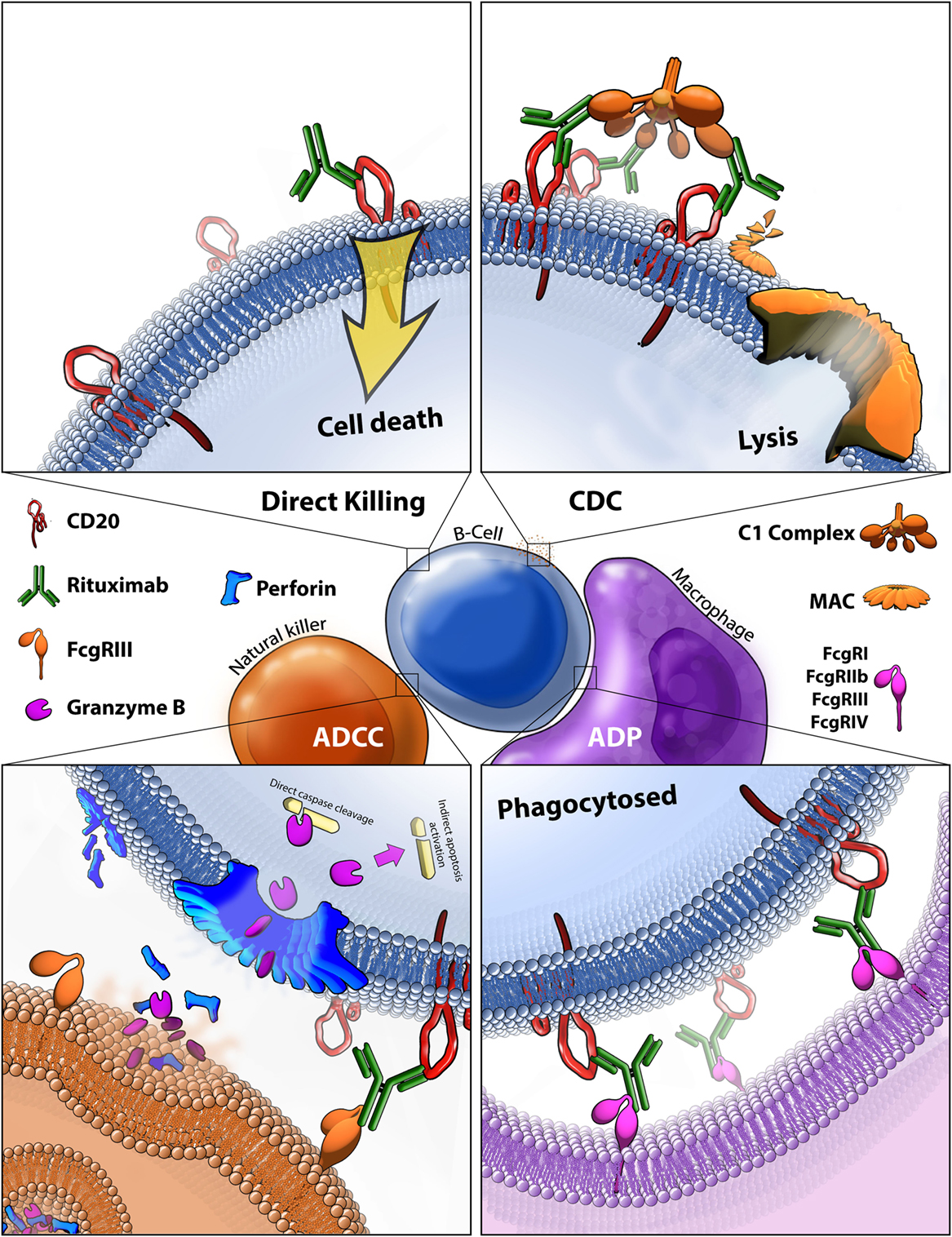
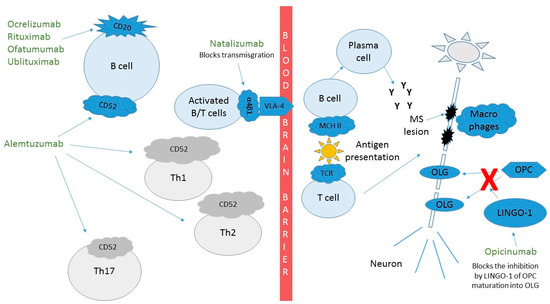
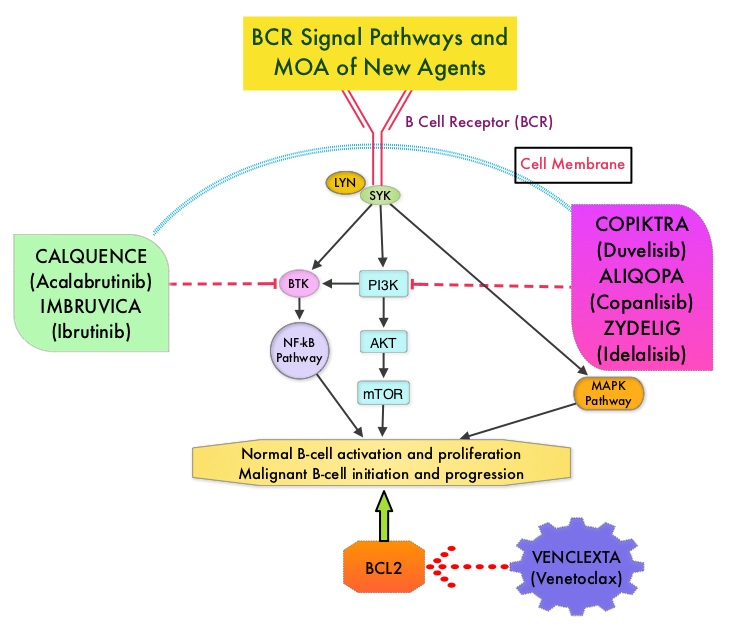
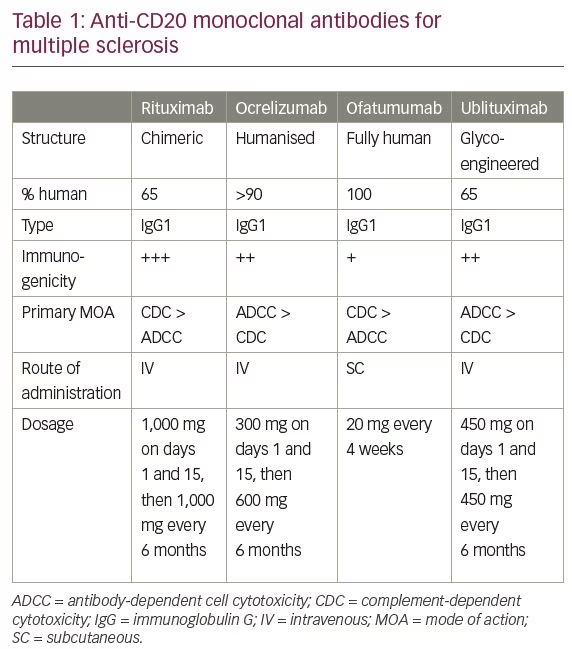
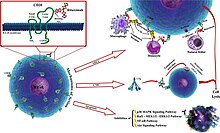
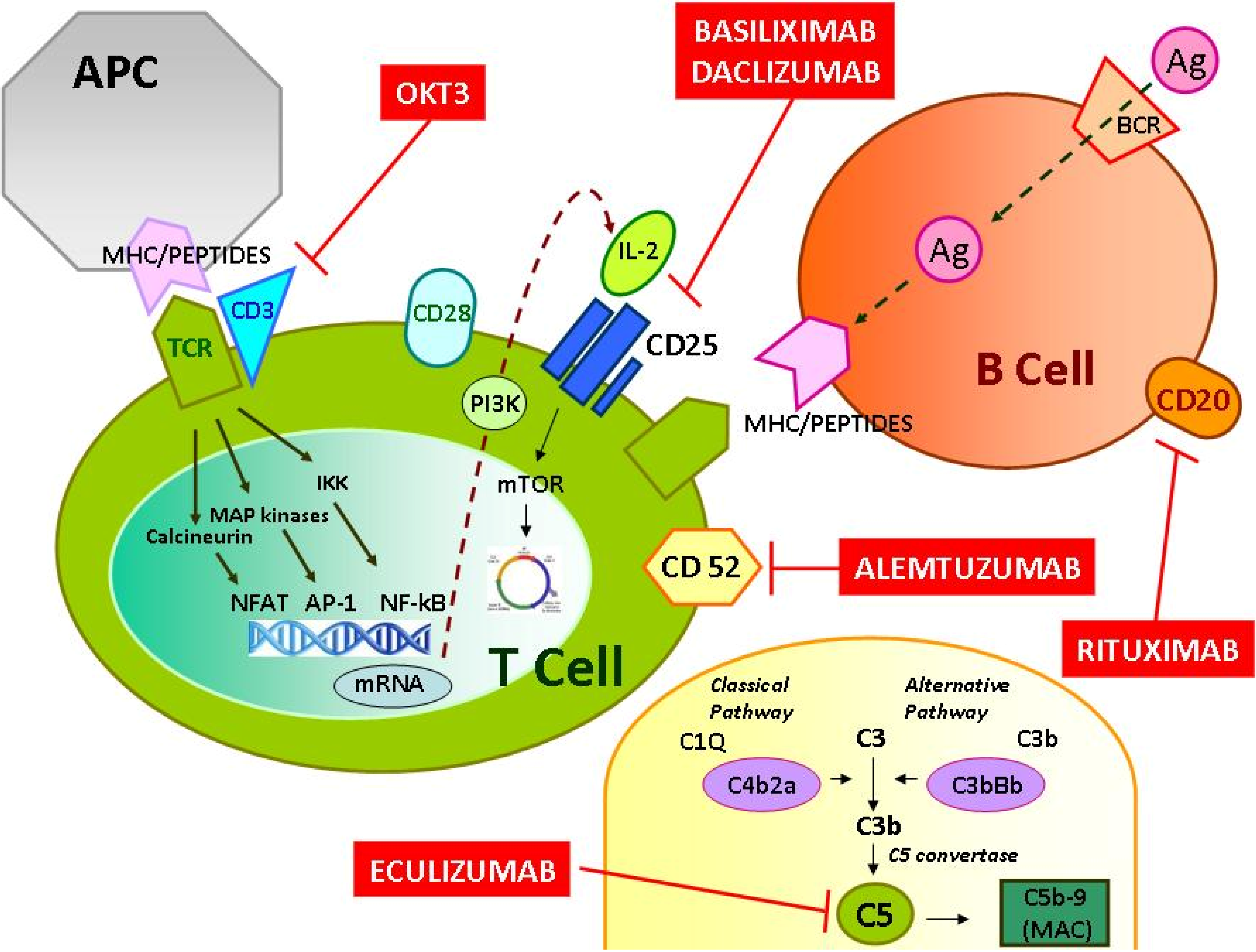
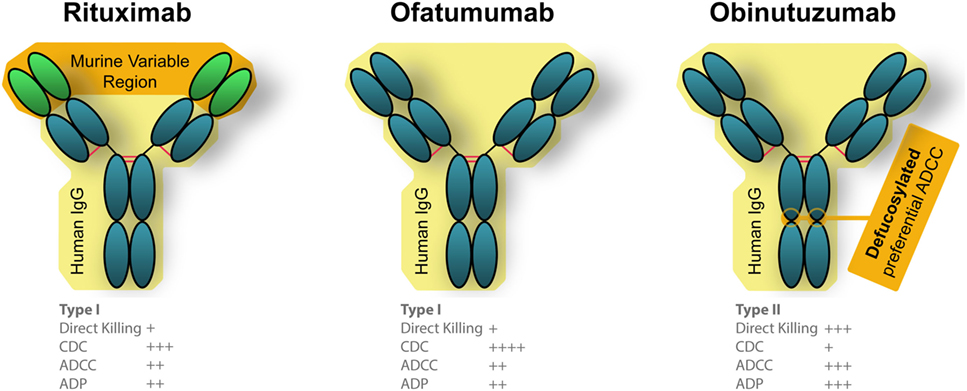
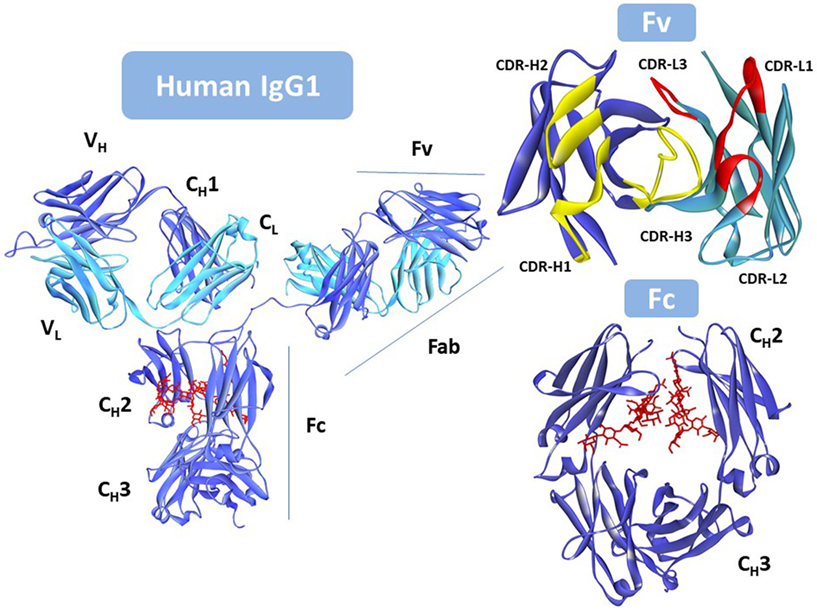
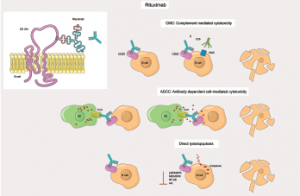
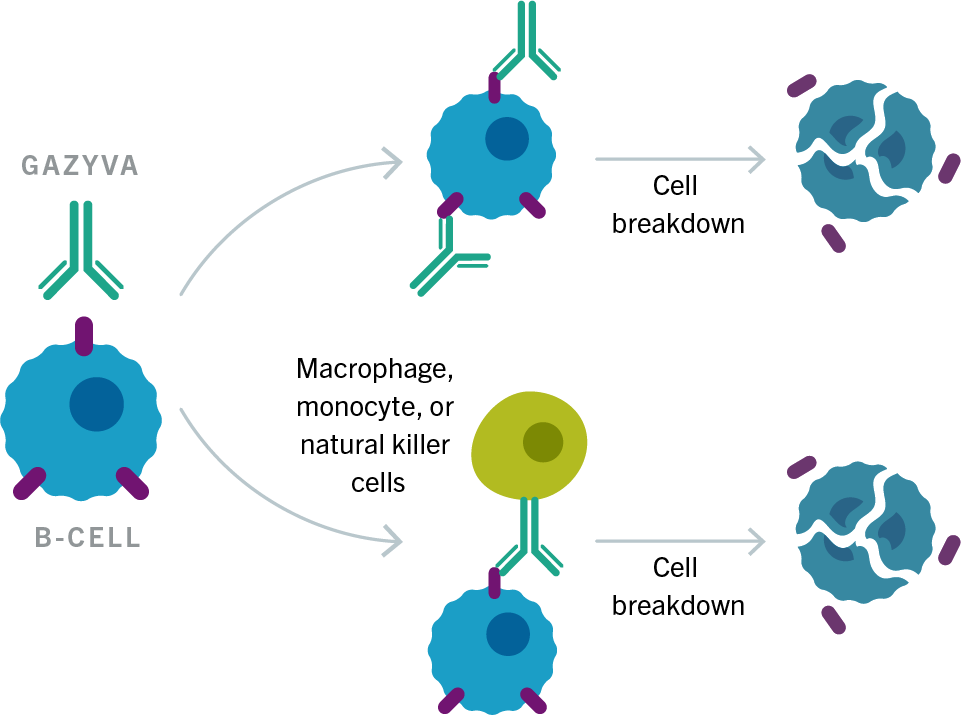
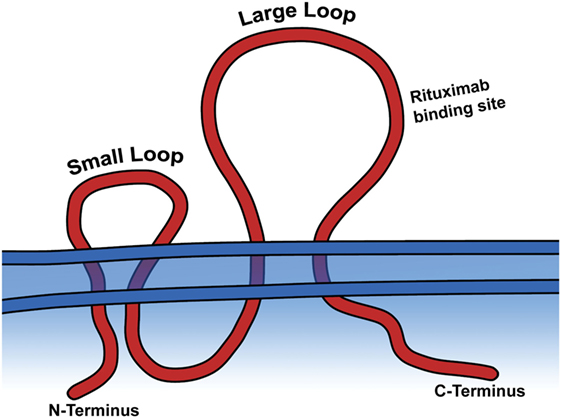
Rituximab moa. Hepatitis b virus hbv reactivation. Rituximab is a chimeric monoclonal antibody against the protein cd20 which is primarily found on the surface of immune system b cells. The antibody is an igg1 kappa immunoglobulin containing murine light and heavy chain variable region sequences and human constant region sequences fda label. Rituximab is a genetically engineered chimeric murinehuman monoclonal antibody directed against the cd20 antigen found on the surface of normal and malignant b lymphocytes.
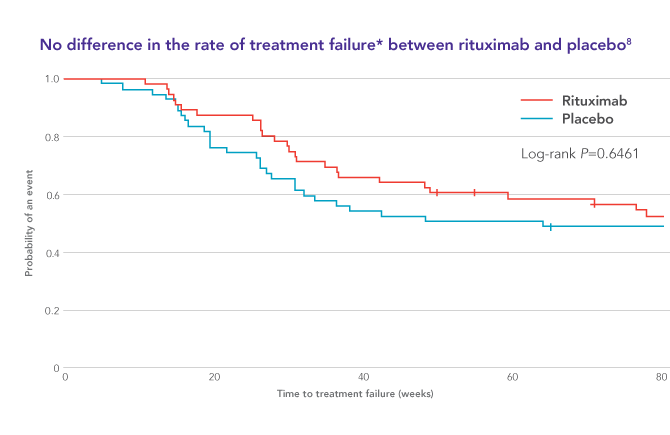
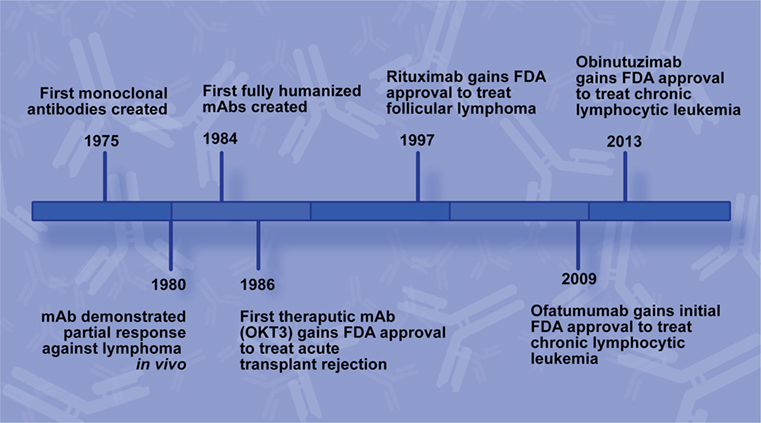
Call your doctor right away if you have problems with speech thought vision or muscle movement. Rituximab was approved for medical use in 1997. In some b cell malignancies rituximab alone can induce high response rates and long term remissions 1 2 while in others adding rituximab to chemotherapy enhances the complete response long term remission and cure rate 3 4. Rituximab may cause a serious brain infection that can lead to disability or death.
Treatment with rituximab at standard weekly dosing is effective in more than 50 of patients with relapsed or refractory cd20 positive follicular non hodgkins lymphoma but is not curative. Rituximab a chimeric monoclonal antibody targeted against the pan b cell marker cd20 was the first monoclonal antibody to be approved for therapeutic use.